BD supports the Cervical Cancer Prevention Week - 2020
"The high mortality rate from cervical cancer globally can be reduced through a comprehensive approach that includes prevention, early diagnosis, effective screening and treatment programs. There are currently vaccines that protect against common cancer-causing types of HPV and can significantly reduce the risk of cervical cancer." 1
-World Health Organisation
TESTING PARADIGMS AND CHALLENGES
Primary HPV screening
-
Evolving guidelines
-
Maintaining resources through transition
-
Growing colposcopy rates requiring improved triage
-
Impact of vaccination on patient management. As vaccination rates increase, genotypes 16 and 18, accounting for more than 70% of invasive cancers, are becoming less prevalent.2-5
-
Adapting to drastic testing volume changes
CE-IVD and FDA approved
The BD SurePath™ Liquid-based Pap Test and the BD Onclarity™ HPV Assay are both CE-IVD marked and approved by the FDA for HPV primary screening, co-testing and cytology primary with ASCUS reflex.
BD – Addressing the challenges in HPV primary screening
The BD Onclarity™ HPV Assay answers the needs for primary HPV screening in the post-vaccination era. It offers extended genotyping that supports risk stratification and persistence monitoring.
The BD COR™ System enhances both laboratory outcomes and patient management with advanced molecular diagnostic capabilities. It offers a solution for high volume labs with a large on-board sample and reagent storage capacity to reduce hands-on and increase walk-away time.6
The BD Viper™ System is a benchtop system ideal for small to medium volume labs.

BD – Addressing the challenges in co-testing
The synergy of the BD Onclarity™ HPV Assay and the BD SurePath™ Liquid-based Pap Test allows high-sensitivity molecular testing and cytology to be performed on a single sample.
Acting as a single source provider for both HPV and cytology testing, BD offers solutions that accommodate the shift in testing volumes.
BD – Addressing the challenges in cytology primary
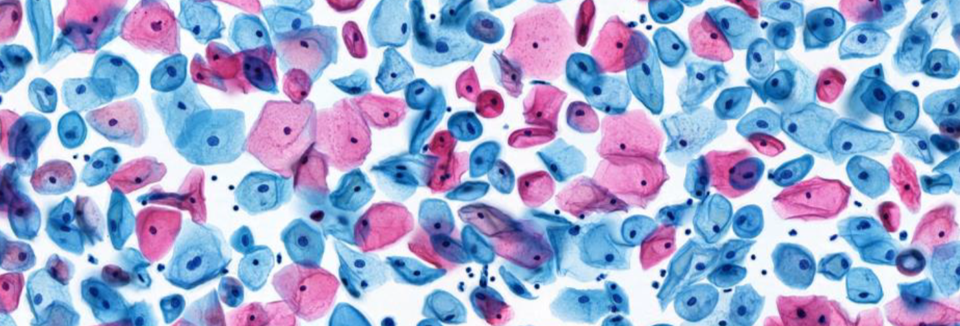
The BD SurePath™ Liquid-based Pap Test offers a simple method to collect cervical samples and sends 100% of the cells to the laboratory. Its proprietary cell enrichment process ensures a consistently lower unsatisfactory results rate. 7,8

BD Symposium EUROGIN 2019
At the EUROGIN 2019 held in Monaco, BD’s contribution included the symposium titled Enhancing patient management and extending the power of HPV testing through genotyping and automation. Mark H Stoler, MD from the University of Virginia spoke about the insights derived into the clinical performance of HPV genotyping from the longitudinal phase of the BD Onclarity™ HPV Assay trial. Jesper Bonde, PhD, from the Copenhagen University Hospital described the evidence behind and the design of algorithms for risk-based primary HPV screening using genotype information. The symposium was chaired by Jeff Andrews, MD, Worldwide Medical Director, Women’s Health and Cancer, BD Life Sciences.
BD will be at IPVC 2020

Visit us at booth #04 and attend our symposium in room 114, at 10:15-11:45 on Thursday, 26th March!
Let's have a conversation






