Protecting photosensitive
medication from light
Did you know that there are a lot of drugs that have to be protected from light?
And for some drugs, exposure to light can have harmful effects on patients?
EXPOSURE OF PRODUCTS TO LIGHT CAN LEAD TO SIGNIFICANT DECOMPOSITION OF THE DRUG1

Decomposition of the drug substance may result in:
- Loss of potency, resulting in loss of effect
- Potential formation of (toxic) degradation products
- Side effects
Decomposition of other formulation components may result in:
- Change in physicochemical properties (eg. viscosity, droplet size...)
- Drug precipitation
THESE DEGRADATIONS CAN CAUSE SIGNIFICANT RISKS TO PATIENT SAFETY SUCH AS1:
- Potential emboli within the blood vessel (i.e. derived from precipitation as result of photochemical reactions)
- Incorrect medication dosage
DID YOU KNOW?
CASE STUDY

Loss of drug potency due to degradation long term infusion:
- Long term infusion (24h)
- 10% of the drug remained intact towards the end of the infusion period
- Patient did not respond to treatment
- Doctor prescribed a higher dose
- New infusion bag with 100% drug was administered to patient
- Potential issues: severe side effects and/or death
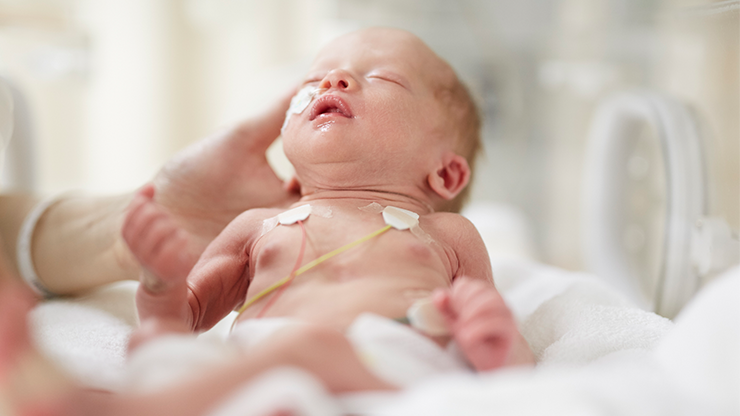
Formation of toxic degradation products in neonates:
- Parenteral nutrition products contain amino acids and/or lipids, multivitamins and trace elements
- Light exposure causes formation of peroxides and other degradation products
- Premature neonates have reduced oxidant defence mechanisms
- Serious adverse effects can occur due to high oxidative stress
KEY FACTORS IN PHOTOSTABILITY ISSUES
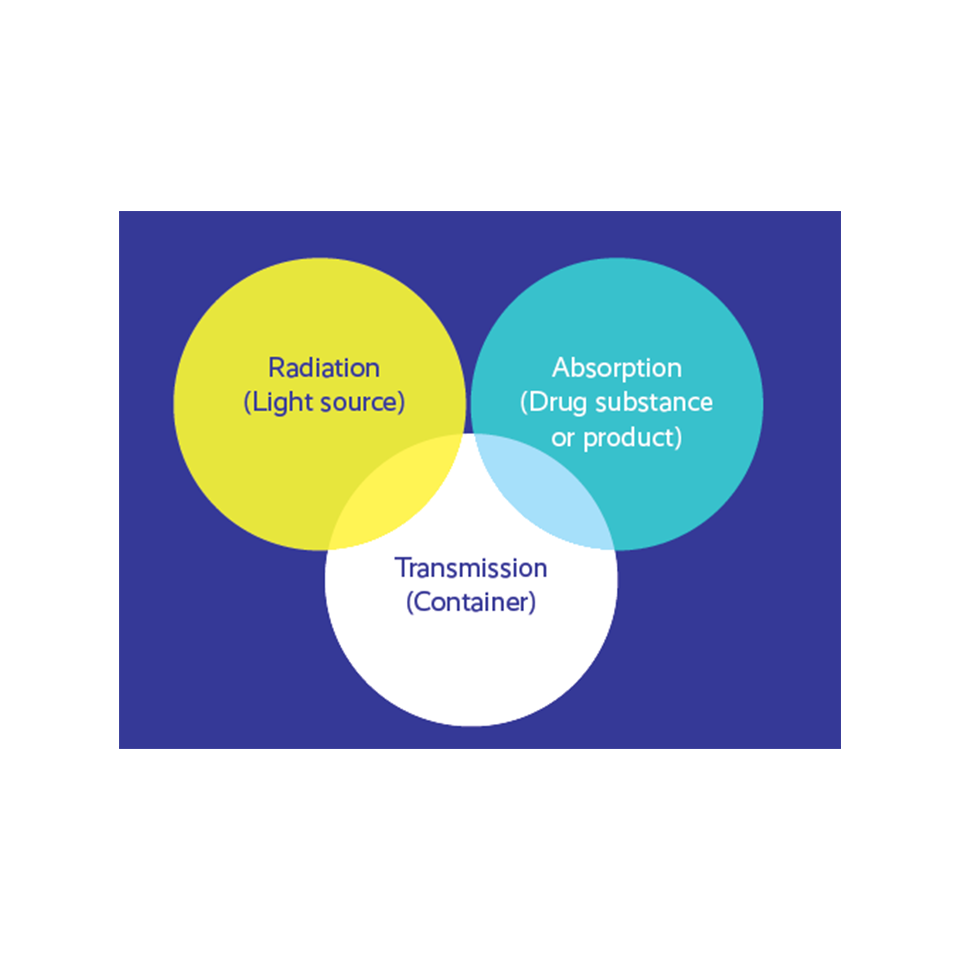
Light radiation sources
Ultraviolet (UV) and visible (Vis) radiation is relevant to photochemical degradation of parenterals.
Major sources: natural sunlight (direct and through a window) and artificial light.
Different sources deliver light at different wavelengths and can affect the same product in different ways.
Absorption of light
Absorption of light is the second key factor in drug photostability issues. Only light that is absorbed by the molecules in the system can induce a photochemical process.
Different materials absorb light at different wavelengths (absorption spectrum).
When there is an overlap between the drug absorption spectrum and the light source’s emission spectrum, the drug molecule gains excess energy.
Transmission of light
The transmission of light through the container is the third key factor in drug photostability.
The product remains “safe” if the container filters out the radiation that can interact with any molecule in the drug formulation.
MEDICATIONS CAN BE EXPOSED TO DIFFERENT LIGHT CONDITIONS FOR DIFFERENT PERIODS
PROTECTING MEDICATION FROM LIGHT DEGRADATION
BD Alaris ™ & BD BodyGuard ™ - protecting photosensitive medication from light in IV settings

- Protect photosensitive medication with our light protected dedicated disposable portfolio.
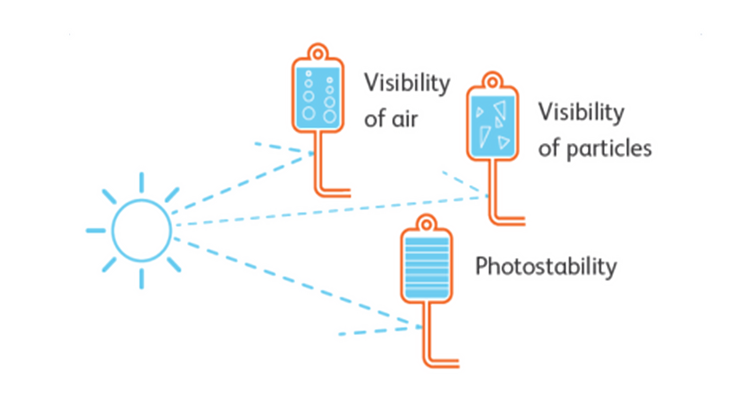
- Drugs that need to be light protected can be administrated by BD light protected (amber) infusion pump sets during infusion therapy.
LEARN MORE
Medicines and light - ensuring patient safety: Photostability of parenteral products
Hanne Hjorth Tønnesen
Register below to have a free copy of the booklet written by Professor Hanne Hjorth Tønnesen on photostability of parental drugs to learn more about this topic.

Download the booklet here!