From discovery to commercialisation, accelerate your CAR-T cell workflow
Through research or clinical development and manufacturing QC, consolidate your cell therapy projects with flexibility, reproducibility and compliance.
From discovery to commercialisation, accelerate your CAR-T cell workflow
Through research or clinical development and manufacturing QC, consolidate your cell therapy projects with flexibility, reproducibility and compliance.
Advance your CAR-T cell projects
From the early steps in exploratory studies to the manufacturing QC process and clinical trials of CAR-T cells, BD Biosciences offers a comprehensive set of solutions that can help maximise your success.
![]()
Discovery Suite
Study CAR-T cells in greater detail with our flow cytometry and single-cell multiomics solutions
![]()
Commercialisation Suite
Benefit from our highly standardised flow cytometry solutions in your clinical trials or during CAR-T manufacturing QC processing
CAR-T Discovery Suite Workflow
Analyse and interpret your valuable data with a comprehensive set of advanced bioinformatics tools for both flow cytometry and SCM including FlowJo®, BD FACSDiva™ and
BD FACSChorus™.
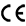
In this webinar, Dr. Fabio Luciani from the UNSW Cellular Genomics Future Institute in Sydney, Australia discusses the workflow that was used in analysing cryo-preserved blood samples from patients undergoing CAR-T cell therapy, featuring BD® AbSeq Technology, its 40 simultaneous markers and the BD Rhapsody™ Single-Cell Analysis System.
From sample preparation, to cell type identification, gene expression analyses and bioinformatics, Dr. Luciani describes his full research process and the systems that supported it.
For Research Use Only. Not for use in diagnostic or therapeutic procedures.
With more than 45 years of experience in single-cell science, our single-cell analysis experts can support you with your sample preparation and antibody panel design workflows.

CAR-T Commercialisation Suite Workflow
The CAR-T Commercialisation Suite
Flow cytometry is a powerful tool to characterise cell phenotypes to define critical quality attributes for cell therapy products such as cell viability, identity, potency and purity.1,3
As well, CE-IVD reagent solutions for immune assessment and minimum residual disease (MRD) monitoring can support you in generating reproducible, consistent data during clinical trials.
Our IVDR* compliant flow cytometer with integrated automation options and assay portability features facilitates handover steps during clinical development and manufacturing QC.
Discover the full range of BD solutions for cell and gene therapy manufacturing QC and clinical trials.
*CE-IVDR: in vitro diagnostic medical device CE-marked in compliance with the European In Vitro Diagnostic Medical Device Regulation (EU) 2017/746.
Support greater standardisation and reproducibility in your workflow with our CE-IVD solutions:
Boost your workflow with bioinformatic solutions that prioritise compliance:

The BD FACSLyric™ Flow Cytometry System, with a series of built-in daily quality control steps, helps ensure reproducible instrument performance supporting accuracy of results. 21 CFR Part 11 features help to drive compliance in controlled environments. Assay portability of user-defined assays simplifies and standardises instrument set-up to facilitate manufacturing expansion to multiple sites.
The BD FACSDuet™ System (BD FACSDuet™ Sample Preparation System and BD FACSDuet™ Premium Sample Preparation System) is designed to enable pre-analytical automation, process standardisation and automated cocktailing, thereby driving efficiency and consistency in workflows. When physically integrated with the BD FACSLyric™ Flow Cytometer, the BD FACSDuet™ System delivers front-to-end automation with walkaway capabilities.
With on-board washing and on-board centrifugation, the BD FACSDuet™ Premium Sample Preparation System further enhances efficiency, flexibility and standardisation of protocols, further reducing hands-on time to meet increasing workload demands.

BD was delighted to partner with CGT Catapult to support their multiple process analytical technologies (PAT) Consortium - a collaboration between leading companies to test novel PATs as applied to an exemplar 8-day CAR-T cell culture bioprocess. BD worked with CGT Catapult to develop a novel, 12-colour flow cytometry panel for the BD FACSLyric™ flow cytometer which was used as a product characterisation step for process QC.
In CAR-T clinical trials, flow cytometry can be used for patient screening and selection.1,3 Immune cell enumeration and phenotypic characterisation can be used to understand cellular kinetics.3 Follow-up assessments can determine MRD in patients with haematological neoplasia, providing information related to the efficacy of the therapy.3,4 CE-IVD solutions including automated gating and analysis support standardisation in multi-site settings and contribute to reproducibility of results.

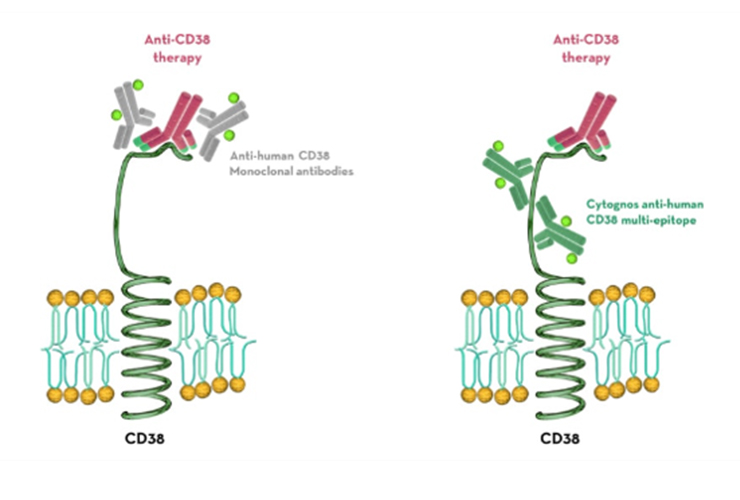
Therapeutic anti-CD38 monoclonal antibodies target CD38 which is highly expressed on the surface of multiple myeloma cells, hampering its detection by flow cytometry.5
The anti-human CD38 multi-epitope reagent contains a mixture of antibodies that improves the identification of CD38 molecules on plasma cells in flow cytometry.

Built on a foundation of excellence and immunological expertise, BD Biosciences is committed to unlocking the potential of flow cytometry-based CDx solutions.
Our dedicated CDx team provides solutions ranging from assay development to CDx commercialisation.
If you would like to learn more about our CDx solutions, please fill out the form below and a member of our dedicated team will reach out to you.
Instrument solutions:
Fill out the form below to access
additional resources for your CAR-T journey