Why recognising primary immunodeficiency is important
The CE-IVD PIDOT Solution
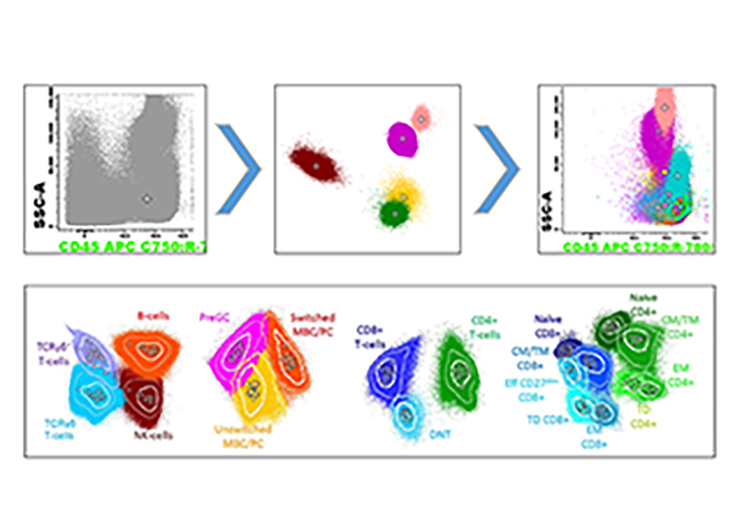
Automated, expert guided Analysis and Gating with BD Infinicyt™ software
Primary immunodeficiencies (PID) are complex and can have various clinical presentations. Because of this complexity, confirmation of PID diagnosis can take time. This delay impacts patient management and can lead to shortened life expectancy.1 Currently 70-90% of Primary Immunodeficiency (PID) cases are assumed to be undiagnosed.1

As PIDs have highly variable presentations, immunodeficiencies initially attributed to secondary causes may hide or be partly caused by underlying PID.2 That’s why, in an era of immune modulating biologicals, it is important to distinguish between PID and SID.2
Flow cytometry is an essential tool for the screening and diagnosis of PID and inborn errors of immunity (IEIs). Inborn defects in the lymphoid system are the most common group in patients with PID. Flow cytometry also has a shorter turnaround time and is less expensive than genetic testing.3
The EuroFlow™ Consortium has developed an algorithm allowing for flow cytometric screening of patients with suspicion of PID in a standardised approach that can guide and prioritise further diagnostic modalities and clinical management.4
The PIDOT (Primary Immune Deficiency Orientation Tube), based on the scientific validation work of the EuroFlow™ Consortium, offers a CE-IVD standardised workflow from sample preparation to automated, expert guided analysis and reporting with BD Infinicyt™ software, including age-specific reference ranges.

Automated, expert guided Analysis and Gating with BD Infinicyt™ software
PIDOT Database Report with age-matched reference values
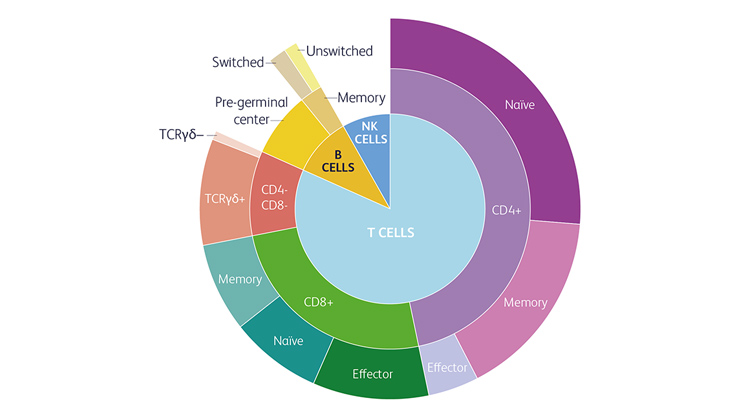
Compared to classical flow cytometry, the Next Generation Flow™ approach offers standardisation and higher sensitivity.4
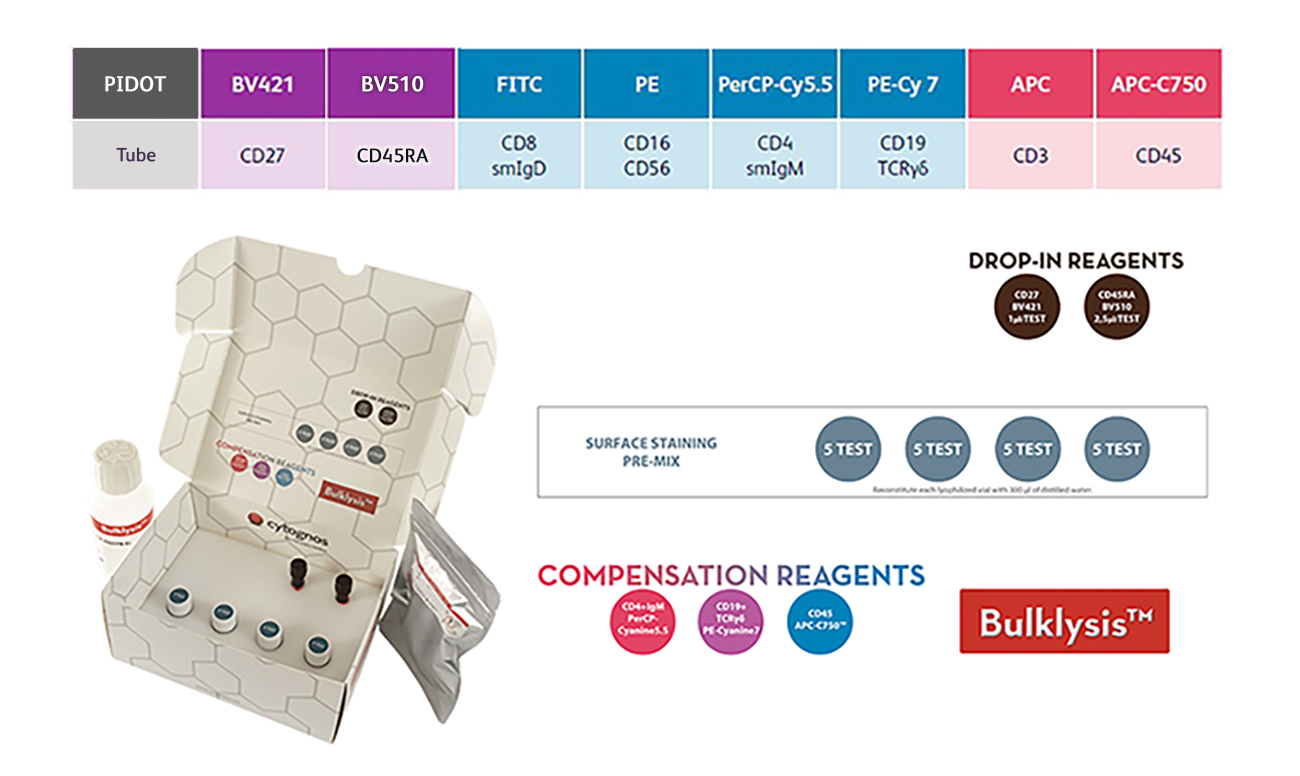
The PIDOT Reagent Kit is designed for the screening of suspected PID using the Next Generation Flow™ approach in peripheral blood specimens. In addition, it can be used for the study of changes in the relative distribution of the different leukocyte populations and subpopulations.
It enables assessment of lymphocyte subsets as listed in ESID (European Society of Immunodeficiency) clinical working criteria.5,6
The Next Generation Flow™ Approach includes standardisation of sample preparation, acquisition and analysis. Its acquisition of at least one million cells enables higher sensitivity compared to classical flow cytometry. This increased sensitivity supports reliable results and is relevant for those lymphocyte populations with lower frequency (Bulklysis™ approach).
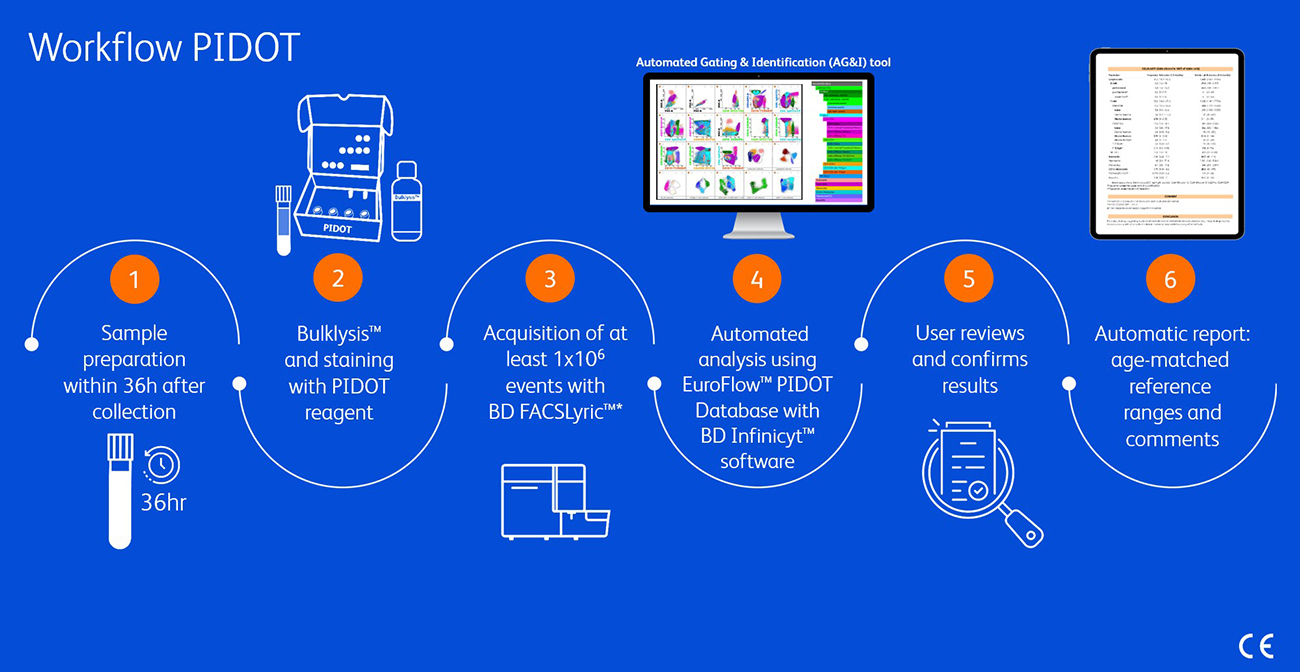
This illustration is not intended to show the complete workflow. For full details please refer to the Instruction for Use for CYT-PIDOT8.7
*CYT-PIDOT8 should be used in combination with the EuroFlow™ Standard Operation Procedures for instrument setup.
By combining B and T cell markers in one CE-IVD panel, changes in the relative distribution of different leukocyte populations and lymphocyte subpopulations can be identified in a first screening step. Pre-mixed lyophilized reagents add additional efficiency.
The automated, expert guided gating and reporting of age-matched reference ranges in combination with BD Infinicyt™ software adds workflow efficiency and reproducibility.
The Automatic Population Separator (APS) provides best cell cluster separation and identifies the markers that are most significant for this separation.
Using the EuroFlow™ PIDOT Database with BD Infinicyt™ software enables automated comparison with age-matched healthy controls and automated reporting.


Automated, expert guided Analysis and Gating with BD Infinicyt™ software

Listen to Dr. Solano, the head of the haematology department at Nuestra Señora del Prado General University Hospital, Talavera de la Reina, Spain explain how he successfully bridged the gap between patients, clinical units, and laboratory through rapid and more accurate PID screening by implementing the PIDOT solution.
Listen to this webinar from Prof. Martín Pérez Andrés, Departamento de Medicina y Servicio de Citometria, Universidad de Salamanca, Spain describing the EuroFlow-based method for detection of lymphoid cells defects and the benefits of a standardised approach in the screening of PID contributing to early PID diagnosis and classification.
Improving understanding of Primary Immunodeficiency (PID) drives earlier detection of PID.
Listen to Prof. Catarina Martins from NOVA Medical School, Lisbon, Portugal, at ESCCA 2022 and hear informative insights into PID classification from the IUIS Committee.
Inborn defects in the lymphoid system are the most frequent group in patients with Primary Immunodeficiency (PID). Flow cytometry is an essential tool for the screening and diagnosis of PID/IEIs.
Listen to Prof. Catarina Martins from NOVA Medical School, Lisbon, Portugal at ESCCA 2022 on how flow cytometry supports analysis of deficiencies in lymphoid compartment.
Listen to Prof. Catarina Martins from NOVA Medical School, Lisbon, Portugal, at ESCCA 2022 and learn about the algorithm EuroFlow has developed for flow cytometric screening and classification of PID of the lymphoid system and the benefits of the Primary Immunodeficiency Orientation Tube.