Looking for a new solution to prevent CSF leaks?

Seal with confidence
Introducing TissuePatchDural™ Surgical Sealant: the next generation of CSF leak prevention
Seal with confidence
Burst Pressure (mmgHg)1
TissuePatchDural™1
136
DuraSeal®2
80
TachoSil®3
46
Hemopatch4
38
Fibrin sealants2
14
Effectiveness of TissuePatchDural™ vs. DuraSeal® in Posterior Fossa surgery8
CSF leak incidence in patients undergoing craniotomy and craniectomy
TissuePatchDural™
2.6% (n=3/115)
DuraSeal®
96.8% patients free of leaks
10.9% (n=5/46)
(P=0,015)
CSF leak incidence in patients undergoing craniectomy
TissuePatchDural™
3.2% (n=2/62)
DuraSeal®
97.4% patients free of leaks related to product
17.9% (n=5/28)
(P=0,008)
Download the TissuePatchDural™ brochure
- Data on file at Tissuemed. Tested in accordance with ASTMF2392
- Campbell P, Bennett S, Driscoll A, Sawhney A. Evaluation of Absorbable Surgical Sealants : In vitro Testing. Medicine. 2005.
- TachoSil® Instructions For Use, section 12.1
- Lewis KM, Kuntze CE, Gulle H. Control of bleeding in surgical procedures: critical appraisal of HEMOPATCH® (Sealing Hemostat). Med Devices (Auckl). 2015;9:1-10. Published 2015 Dec 22. doi:10.2147/MDER.S90591
Seal with confidence
Burst Pressure (mmgHg)1
TissuePatchDural™1
136
DuraSeal®2
80
TachoSil®3
46
Hemopatch4
38
Fibrin sealants2
14
Effectiveness of TissuePatchDural™ vs. DuraSeal® in Posterior Fossa surgery8
CSF leak incidence in patients undergoing craniotomy and craniectomy
TissuePatchDural™
2.6% (n=3/115)
DuraSeal®
96.8% patients free of leaks
10.9% (n=5/46)
(P=0,015)
CSF leak incidence in patients undergoing craniectomy
TissuePatchDural™
3.2% (n=2/62)
DuraSeal®
97.4% patients free of leaks related to product
17.9% (n=5/28)
(P=0,008)
Download the TissuePatchDural™ brochure
- Data on file at Tissuemed. Tested in accordance with ASTMF2392
- Campbell P, Bennett S, Driscoll A, Sawhney A. Evaluation of Absorbable Surgical Sealants : In vitro Testing. Medicine. 2005.
- TachoSil® Instructions For Use, section 12.1
- Lewis KM, Kuntze CE, Gulle H. Control of bleeding in surgical procedures: critical appraisal of HEMOPATCH® (Sealing Hemostat). Med Devices (Auckl). 2015;9:1-10. Published 2015 Dec 22. doi:10.2147/MDER.S90591
Your choice of dural sealant is critical
The most obvious step to prevent CSF leakage is watertight closure of the dura.1 In vitro studies show that most currently available sealants cannot resist physiological intracranial pressure.1
Experts recommend thoroughly assessing any dural sealant before clinical application.1

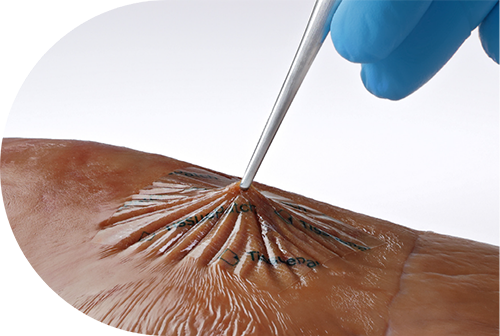
TissuePatchDural™ is thinner than a human hair4 and is designed to bond to dura in 60 seconds with moderate manual pressure*
TissuePatchDural™ seamlessly integrates into the surgical site, resulting in minimal compression. It adheres closely to the dura and can withstand a high burst pressure thanks to TissueBond™ technology. This patented polymer technology reacts with the protein found within the tissue surface to create a covalent bond within 60 seconds.
Advanced surgical sealing is now in your hands
See how TissuePatchDural™ is designed to help you be confident at each step:
Place with confidence
TissuePatchDural™ is transparent
TissuePatchDural™ is only 0.04 mm thick

Offering an unrestricted view of the dura closure
Clear film with visible TissuePatchDural™ logo in Methylene Blue to aid in correct product orientation
Enabling identification that the dural seal is watertight
So you can feel confident as you place it on the dura closure
TissuePatchDural™ is only 0.04 mm thick

Designed for use in confined spaces
May swell slightly to a total thickness of <0.5 mm
Resulting in minimally added compression
So you can feel confident of the seal after you place it

*In pre-clinical models
Let's have a conversation