Battling Surgical Site Infections together
Committed to sustainable healthcare
Battling Surgical Site Infections together
Committed to sustainable healthcare
Surgical Site Infections (SSIs) are prevalent and remain an important topic for healthcare systems around the world. The facts speak for themselves:
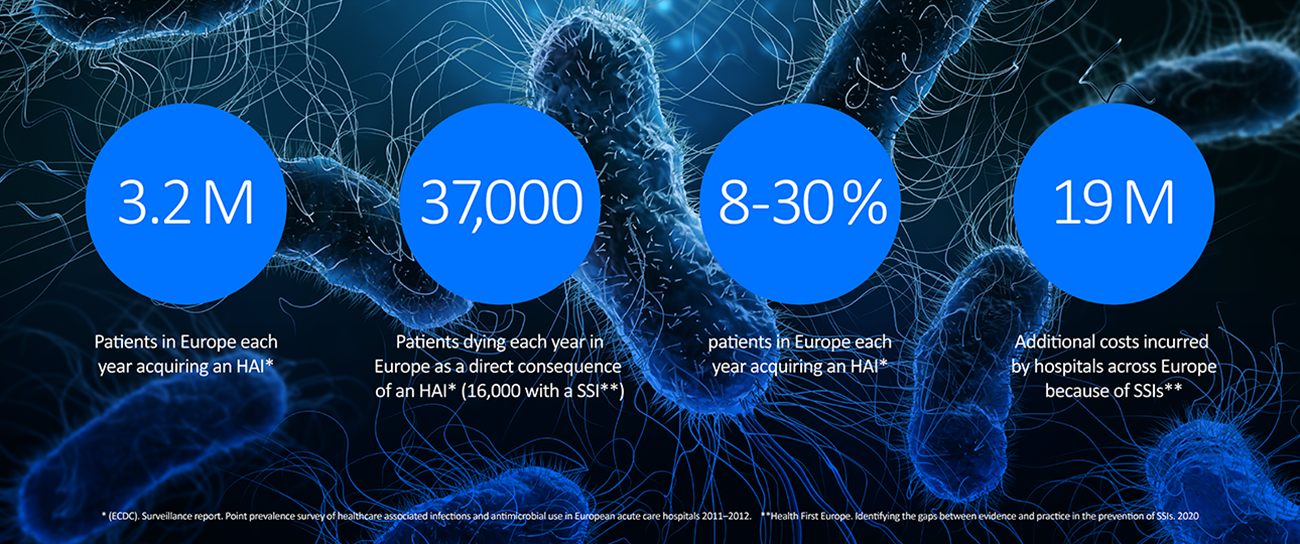
SSIs impact patient recovery, quality of life and impose a substantial financial and personal burden.* At BD, we strive every day to work collaboratively with our partners to alleviate the burdens caused by SSIs.
Work with BD to discover the latest evidence-based strategies to fight SSIs.
*R. Royle, B.M. Gillespie, W. Chaboyer et al. Journal of Infection and Public Health 16 (2023) 792-798.
SSIs depend on many factors, however over 50% of them could be prevented using current evidence-based strategies.*
At BD we are proud to partner with HCPs who have made patient safety and SSI prevention a priority.
It takes commitment, effort and teamwork, but with passion and dedication our customers, at all levels of the hospital, are engaged in the fight against SSIs.
Meet some of them and listen to their stories.
*Berríos-Torres SI, et al. Healthcare Infection Control Practices Advisory Committee. Centers for Disease Control and Prevention Guideline for the Prevention of Surgical Site Infection, 2017. JAMA Surg. 2017 Aug 1;152(8):784-791.
While the ideal antiseptic should be broad spectrum to eliminate as much skin pathogens as possible, it should also be safe from contamination.
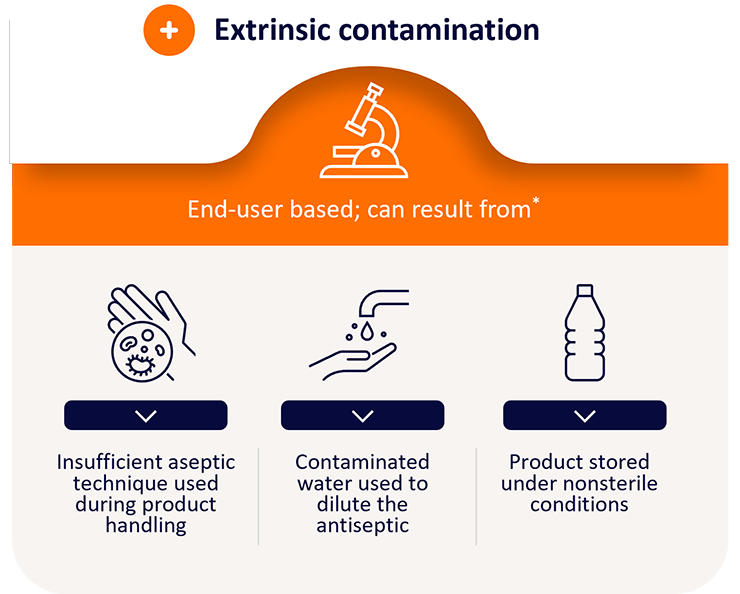
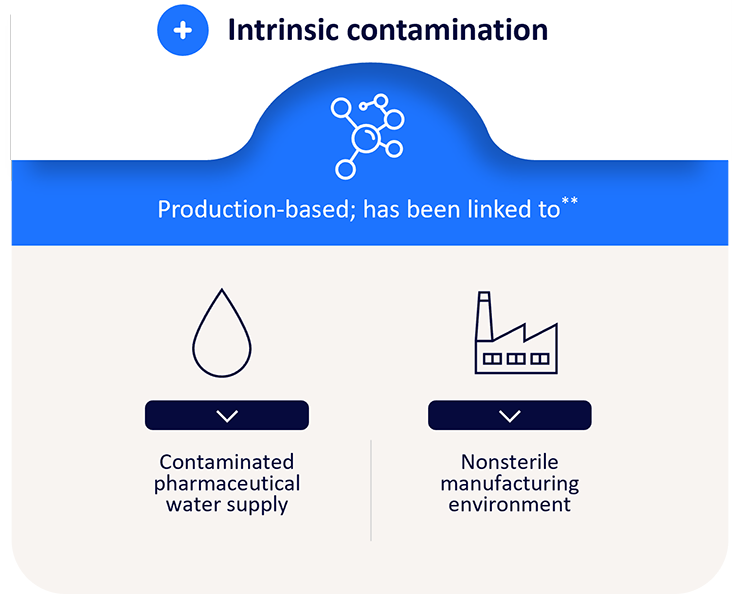
*Research C for DE and. Questions and Answers: FDA requests label changes and single-use packaging for some over-the-counter topical antiseptic products to decrease risk of infection. FDA. Published online November 3, 2018. https://www.fda.gov/drugs/drug-safety-and-availability/questions-and-answers-fda-requests-label-changes-and-single-use-packaging-some-over-counter-topical
Research C for DE and. FDA Drug Safety Communication: FDA requests label changes and single-use packaging for some over-the-counter topical antiseptic products to decrease risk of infection. FDA. Published online June 21, 2019. https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-requests-label-changes-and-single-use-packaging-some-over-counter
**Wiemken, T.L. Skin antiseptics in healthcare facilities: is a targeted approach necessary?. BMC Public Health 19, 1158 (2019). https://doi.org/10.1186/s12889-019-7507-5
No matter how good the procedural outcome is, if patients get an infection, the overall surgery is impacted.
Lilian sees the people behind the numbers and is not willing to compromise. This is all about teamwork and resilience.
Vicky values quality of care. As a nurse, she thrives on the frontline to make sure all staff communicate and embody a culture of safety.
Please find a summary of Prof. Boermeester's paper below:
Click on the image below to view our infection prevention portfolio.
BD has developed ChloraPrep™, a unique product designed for safety from start to finish.

BD has developed a proprietary process to sterilize the fragile CHG molecule, with an SAL of 10-6, minimizing the risk of intrinsic contamination.

2%CHG/70%IPA recommended by or compliant with latest guidelines*

The solution is embedded in a single use sterile applicator - minimizing manipulation and the risk of extrinsic contamination.
* CDC: Center for Disease Control and Prevention. KRINKO: Commission for Hospital Hygiene and Infection Prevention NICE: National Institute for Health and Care Excellence. SF2H: French Society for Hospital Hygiene. WHO: World Health Organisation.
See full product labeling for complete Instructions For Use and important safety information.

Let's continue the conversation