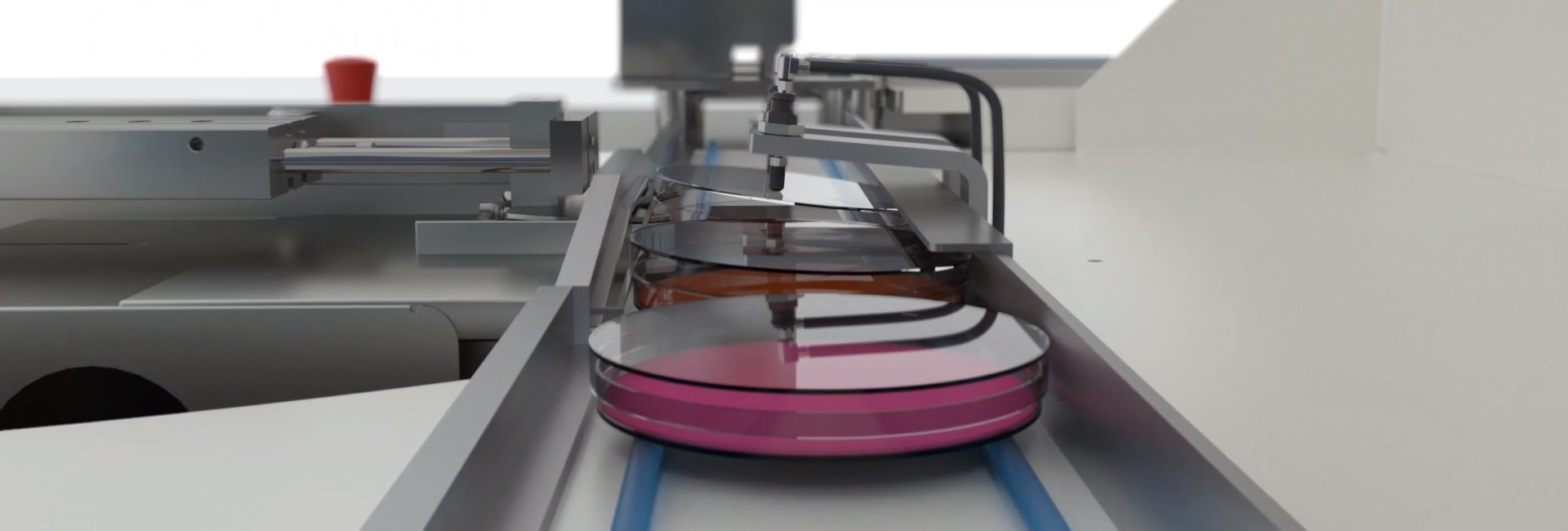
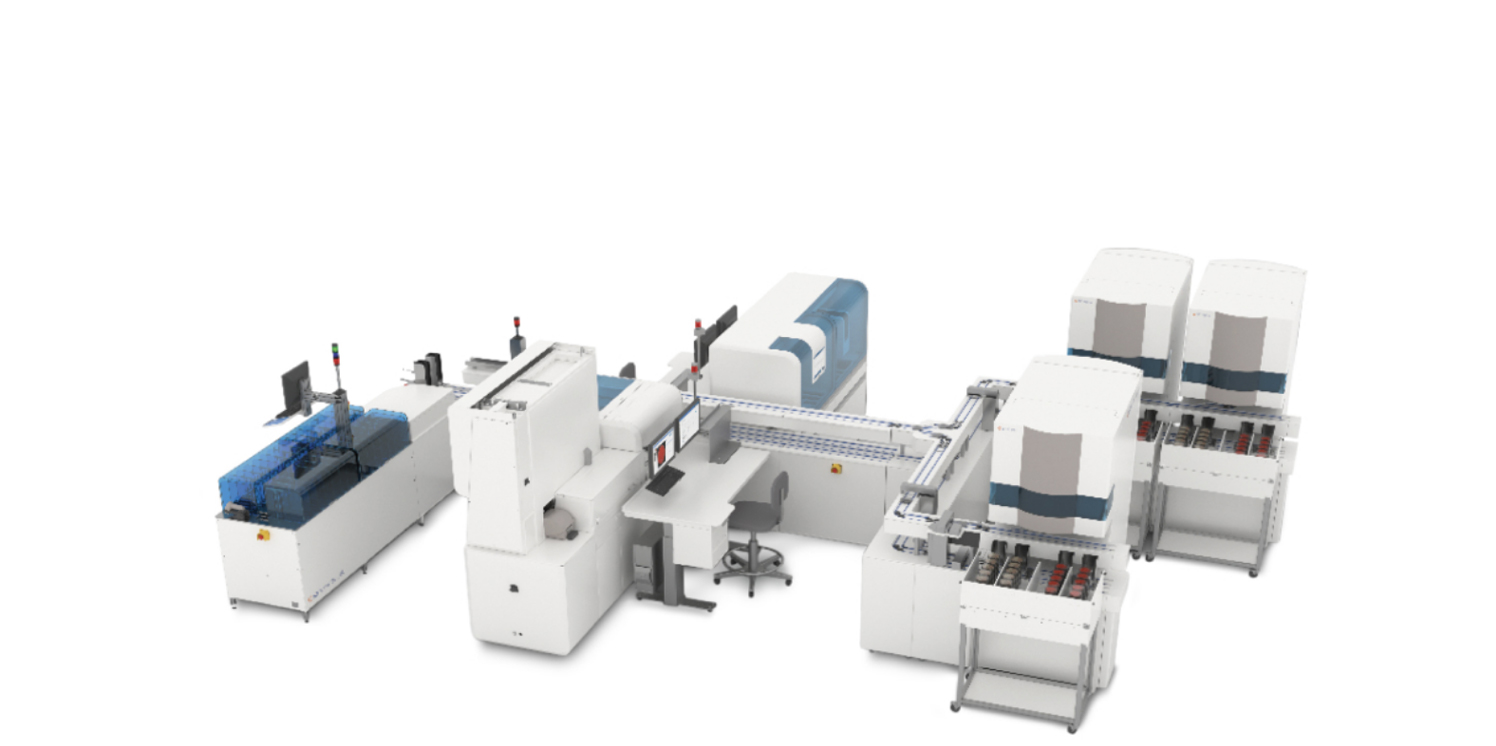
Elevate to modular automation with BD Kiestra™ solutions can help you
to enhance laboratory operations, maximise financial efficiencies, and advance laboratory outcomes1,2,3
Improve staff efficiency,3 reduce risk of errors,1,3 generate accurate results faster2,4 and adapt to testing volume growth.1
Manage labour costs and laboratory expenditures.2
Improve end-to-end quality and accuracy for more confidence in treatment guidance and positive laboratory outcomes.1,5,6
Discover our latest BD Kiestra™ solutions
BD Kiestra™ InoqulA
THE NEXT GENERATION AUTOMATED SOLUTION FOR LIQUID AND NON-LIQUID SAMPLE PROCESSING
Learn more
BD Kiestra™ InoqulA is a comprehensive automated solution for sample processing, delivering workflow efficiency with continuous loading of samples and plates complete traceability with automated plate and slide and informatics connectivity
- The walkaway automated solution that streamlines workflow by processing liquid and non-liquid sample types1
- Allows you to process samples using barcoded plates1
- Reduces the need for subculturing and shortens the time to identification and antimicrobial susceptibility testing2,4,5
- Decreases the risk of false negatives during inoculation with the help of positive dispense verification (PDV) technology
- Standardisation inoculation volume and streaking, enabling fast pathogen identification2,4,5
- 3 workflows: fully automated, semi-automated and manual
- Powered by BD Synapsys™ informatics solution, a single, advanced platform with an intuitive, personalized .user interface designed to support diagnostic workflows
BD Kiestra™ ReadA
INTELLIGENT INCUBATION AND IMAGING ACQUISITION
Learn more
BD Kiestra™ ReadA combines closed door incubation and high throughput imaging to provide standardised and efficient incubation and image acquisition to enable digital imaging.
- Provides constant incubation conditions to enhance the growth of all bacteria including rarely isolated organisms7
- Can increase bacterical isolation by up to 31% compared to traditional microbiology*7
- BD Kiestra™ imaging aplicatons are designed to provide diagnostically relevant standardised images for interpretation by laboratory and timely results
- Digital imaging powered by BD Synpasys™ microbiology informatics solution
* Based on studies of urine cultures with selected organisms
BD Kiestra™ IdentifA
AUTOMATING AND INNOVATING THE MALDI-TOF AND SUSCEPTIBILITY
Learn more
BD Kiestra™ IdentifA automatically pick user selected colonies and creates a single suspension for both MALDI-TOF identification and antimicrobial susceptibility testing.
- Ensures secure identification identification and susceptibility results by utilising the same colony for both MALDI-TOF and antimicrobial susceptibility testing
- Ensure unique workflow and support for routine and challenging isolate, including mucoid strains or low growth organism through adapted protocols.
- Powered by BD Synpasys™ informatics solution
Choose from multiple BD Kiestra™
standalone solutions
Create your own automated lab configuration or adopt BD Kiestra Total Lab Automation (TLA) System
![]() BD Kiestra™ standalone solutions
BD Kiestra™ standalone solutions

BD Kiestra™ InoqulA™
Automates liquid and non-liquid sample processing
- Continuous loading of samples
- Accurate, real-time sample dispense confirmation5
- Priority sample processing
- Rolling bead techology
- Positive dispense verification (PDV) technology
- 3 workflows: fully automated, semi-automated and manual

BD Kiestra™ ReadA
Intelligent incubation and imaging acquisition
- Standardised incubation times and conditions8
- High-resolution (25 mp, telecentric camera) and standardised imaging (BD Kiestra™Optis™ Technology)
- High throughput ensured by 4 tracks performing all activities in parallel
- Assisted culture reading using intelligent algorithms

BD Kiestra™ Urine Culture App
Accurate and timely plate interpretation impacting patient management
- Real-time automatic results reporting
- Growth quatitation
- No growth/non-significant growth reporting
- Early growth reporting

BD Kiestra™ IdentifA
Automates the preparation of a single suspension testing
- Precise colony picking ensured by tips calibration and colony detection
- Active mucoid strain detection and processing
- Single-suspension creation for identification and susceptibility testing
- Unique layering technology to proceed low growth colonies

BD Bruker MALDI Biotyper™
Accelerates identification testing results
- Rapid and accurate pathogen identification8
- Reduce time to appropriate therapy for improved patient management8

BD Synapsys™ informatics
A single, advanced platform with an intuituve, personalized user interface
- Complete culture and patient overview
- Full traceability of workflow
- Timely reporting of actionable results
- Analytics and data-driven reports
![]() BD Kiestra™ Total Laboratory Automation
BD Kiestra™ Total Laboratory Automation
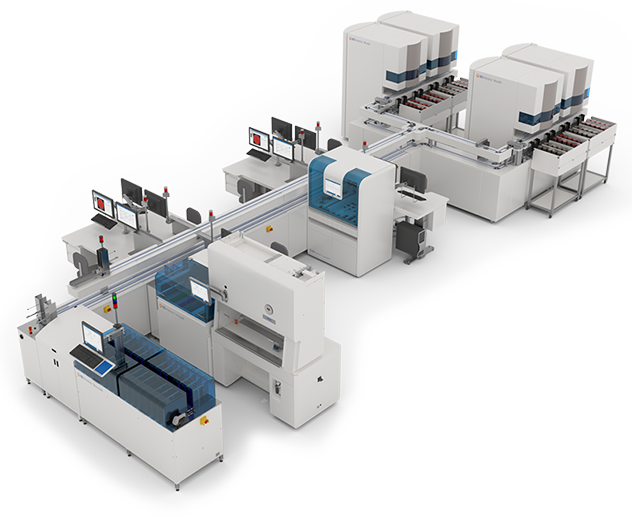
BD Kiestra™ Total Lab Automation
A complete microbiology laboratory automation solution
- Storage capacity of up to 6,912 plates
- 2-way track system connecting automated plating to incubation and to integrated workstations
- Less lab space required for a higher throughput9
1. Page N. Transforming a Canadian microbiology laboratory: laboratory automation and lean processes reduce errors, improve standardisation and result quality while improving productivity. BMJ Qual Saf. 2015;24(11):718-740. 2. Croxatto, A., Dijkstra, K., Prod’hom, G. & Greub, G. (2015). Comparison of Inoculation with the InoqulA and WASP Automated Systems with Manual Inoculation. Journal of Clinical Microbiology, vol. 53 (7), 2298 – 2307 3. Yue P, Zhou M, Zhang L, et al. Clinical Performance of BD Kiestra InoqulA Automated System in a Chinese Tertiary Hospital. Infect Drug Resist. 2020;13:941-947. Published 2020 Apr 1. doi:10.2147/IDR.S245173 4. Graham M, Tilson L, Streitberg R, Hamblin J, Korman TM. Improved standardization and potential for shortened time to results with BD Kiestra™ total laboratory automation of early urine cultures: A prospective comparison with manual processing. Diagn Microbiol Infect Dis. 2016 Sep;86(1):1-4. doi: 10.1016/j.diagmicrobio.2016.06.020. Epub 2016 Jul 2. PMID: 27422083. 5. Iversen et al. Comparative evaluation of inoculation of urine samples with the Copan WASP and BD Kiestra InoqulA instruments. J Clin Microbiol. 2016;54(2):328-332. 6. Ledeboer N et al. The automated clinical microbiology laboratory: fact or fantasy? J Clin Microbiol. 2014;52(9):3140-3146 7. Klein S et al. Significant increase in cultication of Gardernerella vaginalis, Alloscarovia omnicolens, Actinotigunum schallii, and Actinomyces spp. in urine samples with total laboratory automation, Eur J Clin Microbiol Infect Dis. 2018;37(7):1305-1311. 8. Theparee T et al. Total laboratory automation and matrix-assisted laser desorption ionization–time of flight mass spectrometry improve turnaround times in the clinical microbiology laboratory: a retrospective analysis. J Clin Microbiol. 2018;56(1):1-8. 9. Thomson RB Jr, McElvania E. Total Laboratory Automation: what is gained, what is lost, and who can afford it? Clin Lab Med. 2019;39(3):371-389.